— This article by Jerry Cates was first published on 23 October 2015, and revised last on 11 September 2016. © Budsinthenews Vol. 6:10(2).
The woody perennial evergreen herb we know as rosemary (Rosmarinus officinalis) has green needle-like leaves that resemble those of hemlock, and white, pink, purple, or blue flowers. It is a member of the mint family (Lamiaceae).
A native of the Mediterranean region, its name is from two Latin roots that in combination mean “dew of the sea” is formed from the combination of two Latin nouns: ros, “dew” and marinus, “sea.” In some locales it is known by the ancient Greek word ἄνθος (AN-thoze), which means “flower”.
A hardy plant, rosemary is easily cultivated and resists a broad range of pests. Surprisingly adaptable to cold and drought, it is suitable as an ornamental shrub throughout a range of climates. It prefers friable, well-drained soils. Erect shrubs can grow to heights of 2 meters (6.5 feet). The needle-like leaves, which grow to 2–4 centimeters (0.8–1.6 inches) long but only 2–5 mm across, are a glabrous and green dorsally, with pubescent white underparts. Flowers emerge in spring and summer in temperate regions, but bloom constantly in tropical climes.
Extracts of rosemary leaves and stems yield the phytochemicals rosmarinic acid, camphor, caffeic acid, ursolic acid, betulinic acid, and the antioxidants carnosic acid and carnosol.
Rosmarinic acid
![Rosmarinic Acid, (2R)-2-[[(2''E'')-3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]]oxy]-3-(3,4-dihydroxyphenyl)propanoic acid, has the chemical formula C18H16O8, is a caffeic acid ester of salvianic acid A (3,4-dihydroxyphenyllactic acid).](https://budsinthenews.info/wp-content/uploads/2016/04/Rosmarinic_acid-300x143.png)
Rosmarinic Acid, (2R)-2-[[(2”E”)-3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]]oxy]-3-(3,4-dihydroxyphenyl)propanoic acid, has the chemical formula C18H16O8; it is a caffeic acid ester of salvianic acid A (3,4-dihydroxyphenyllactic acid).
As a result of the health promoting and medicinal values of rosmarinic acid, comparative analyses have been conducted to determine the best sources available. One such study of the rosmarinic acid content of various herbs and plants (Shekarchi, et al., 2012), produced the surprising revelation that — while dried rosemary herb contained 7.2 mg/g — spearmint (Mentha spicata), contained more than eight times as much (58.5 mg/g).
Though spearmint had the highest content of rosmarinic acid of all the herbs that were tested in that study, many others also were found to contain considerably more than rosemary. For example, sage (Salvia officinalis) had 5.5x (39.3 mg/g), lemon balm (Mellisa officinalis) 5 x (36.5 mg/g), lemon thyme (Thymus citriodorus) and the Russian sage Perovskia artemisioides 4.4x (31.5 and 31.3 mg/g, respectively), and peppermint (Mentha piperita) 5x (28.2 mg/g).
Other Chemical Constituents
In a study by Ozcan & Chalchat, published in 2008, the essential oil of the dried above-ground parts of wild rosemary collected in Konya, Turkey was analysed by gas chromatography and gas chromatography-mass spectrometry. The oil, which was obtained by hydrodistillation, and was comprised of monoterpenic hydrocarbons, oxygenated monoterpenes and sesquiterpene hydrocarbons, represented 1.9% of the herb. Twenty recognized compounds were identified, including p-cymene (44.02%), linalool (20.5%), gamma-terpinene (16.62%), thymol (1.81%), beta-pinene (3.61%), alpha-pinene (2.83%) and eucalyptol (2.64%).
p-Cymene
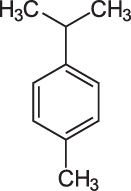
p-Cymene, 1-Methyl-4-(1-methylethyl)benzene, has the chemical formula C10H14, it is and alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group.
p-Cymene, a prominent constituent of essential oil extracted from dried rosemary herb, is a naturally occurring alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group [i.e., an alkyl (of or denoting a hydrocarbon radical) derived from an alkane (a saturated hydrocarbon consisting only of hydrogen and carbon atoms wherein all bonds are singular) as a consequence of the removal of a hydrogen atom derived from methane, containing one carbon atom bonded to three hydrogen atoms, thus CH3) and an isopropyl group [a propyl (a linear three-carbon alkyl substituent, with chemical formula CH2CH2CH3, obtained by removing one hydrogen atom attached to the terminal carbon of propane {a three-carbon alkane with the molecular formula C3H8}with a group attached to the secondary carbon].
There are two less common geometric isomers. o-cymene, in which the alkyl groups are ortho-substituted, and m-cymene, in which they are meta-substituted. p-Cymene is the only natural isomer. All three isomers form the group of cymenes.
p-Cymene is insoluble in water, but miscible with ethanol and diethyl ether. It is absorbed through the skin: studies using (14)C-labelled p-cymene observed a rate of penetration of 254 ug/sq cm over a 60 minute interval.
According to one authoritative study (Matsumoto, T., et al. 1992. Chem Pharm Bull (Tokyo) 40 (7): 1721-6) of the rate of metabolism of p-cymene in rats and guinea-pigs following intragastric or inhalation dosage (100 mg/kg), urinary metabolite excretion was nearly complete within 48 hr, amounting to 60-80% dose. In inhalation experiments 18 urinary metabolites were detected and identified and, of these, rats did not excrete two and guinea-pigs did not excrete a third. No ring-hydroxylation of p-cymene was detected in rats, but guinea-pigs formed small amounts of carvacrol and hydroxycarvacrol. Oxidation of both the methyl and isopropyl groups of p-cymene occurred extensively in both species. The following types of metabolites were formed: monohydric alcohols, diols, mono- and di-carboxylic acids and hydroxyacids. Conjugation with glycine of the cumic acid formed was extensive in guinea-pigs.
Xie, G., et al., in a study published in 2012 (Molecules 17 (7): 8159-73), tested the hypothesis that p-cymene can attenuate acute lung injury (ALI) induced by lipopolysaccharide (LPS) in vivo, and confirmed a protective effect on LPS-induced ALI in mice. In the mouse model, intraperitoneal preconditioning with p-cymene resulted in a significant reduction of pro-inflammatory cytokines (TNF-alpha, IL-1beta and IL-6), lung water gain, inflammatory cell infiltration, and lung tissue myeloperoxidase activity. Furthermore, p-cymene blocked the phosphorylation of IkBalpha protein and mitogen-activated protein kinases (MAPK) signaling pathway activation. Histopathologic examination of lung tissue indicated that p-cymene treatment markedly decreased focal thickening, congestion, pulmonary edema, and inflammatory cells infiltration.
Another study (Zhong, W. et al. 2013; Inflammation 36 (3): 529-37), investigated the effects of p-cymene on lipopolysaccharide (LPS)-induced inflammatory cytokine production, both in vitro and in vivo, and produced evidence that p-cymene has potential anti-inflammatory effects on cytokine production. The production of tumor necrosis factor-a (TNF-a), interleukin-1beta (IL-1beta), interleukin-6 (IL-6), and interleukin-10 (IL-10) in LPS-stimulated RAW 264.7 cells and C57BL/6 mice was evaluated by sandwich ELISA, and the mRNA levels of cytokine genes were examined in vitro by semiquantitative RT-PCR. A further study analyzed the activation of nuclear factor-kB (NF-kB) and mitogen-activated protein kinase (MAPK) signaling pathways by western blotting. It was discovered that p-cymene significantly regulated TNF-alpha, IL-1beta, and IL-6 production in LPS-stimulated RAW 264.7 cells and downregulated the levels of relative mRNAs. In in vivo trail, p-cymene suppressed the production of TNF-a and IL-1beta, increased IL-10 secretion, and inhibited LPS-induced activation of extracellular signal receptor-activated kinase 1/2, p38, c-Jun N-terminal kinase, and IkBalpha.
Linalool
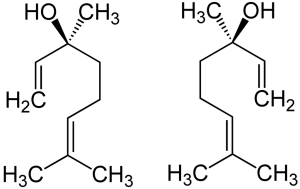
Linalool’s two stereoisomers: (S)-(+)-linalool (left) is known as licareol, and (R)-(–)-linalool (right), is known as coriandrol.
Another prominent constituent of the essential oil extracted from dried rosemary herb, linalool is a naturally occurring terpene alcohol that is also found in many other flowers and spice plants. This terpene is best known for its floral, spicy scent. Thus it is used extensively in commercial air fresheners and similar applications, where it may identified in the product label under one of the following synonyms: β-linalool, linalyl alcohol, linaloyl oxide, p-linalool, allo-ocimenol, and 3,7-dimethyl-1,6-octadien-3-ol.
Two enantiomeric stereoisomers exist for linalool, both of which are found in nature. Enantiomers are stereoisomers related by reflection: i.e., like their human hand macro-analogs, they are mirror images that cannot be superimposed. Every stereogenic center in one has the opposite configuration in the other. Enantiomers of a compound possess the same physical constituents. However, the fact that they rotate polarized light in different directions and interact differently with various optical isomers of other compounds often leads to distinct biological effects. Pure enantiomers also exhibit the phenomenon of optical activity and can be separated only with the use of a chiral agent. In nature, only one enantiomer of most chiral biological compounds, such as amino acids (except glycine, which is achiral), is present.
The enantiomeric form (S)-linalool is a major constituent of the essential oil extracted from the seed of coriander (Coriandrum sativum), and from the flowers of palmarosa (Cymbopogon martinii var martinii), and sweet orange (Citrus sinensis). (R)-linalool is a constituent of lavender (Lavandula officinalis), bay (Laurus nobilis), sweet basil (Ocimum basilicum), and others.
Each of linalool’s enantiomers evoke distinct neural responses in humans. (S)-(+)-Linalool is perceived as sweet, floral, greenish woody orange smell, and has an odor threshold 7.4 ppb. The (R)-form, by contrast, tends toward a woody lavender-like fragrance, and — with an odor threshold 0.8 ppb — takes only about 1/10th as much to evoke a neural response.
Gamma-terpinene
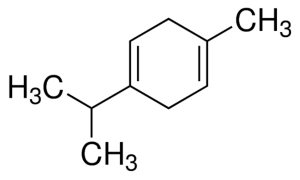
Gamma terpinene. Note the similarity to p-cymene, and to linalool. This same structure is also similar to that of carvacrol and its isomeric version thymol, both of which are derived from p-cymene by the addition of a hydroxyl group.
Within the large, diverse class of organic compounds known as terpenes are four isomeric hydrocarbons known as terpinenes. Each has the same molecular formula and carbon framework, but differ in the position of carbon-carbon double bonds. α-Terpinene (4-Methyl-1-(1-methylethyl)-1,3-cyclohexadiene), is found in nature and has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene (4-Methylene-1-(1-methylethyl)cyclohexene) is not found in nature, but can be prepared synthetically from sabinene. γ-Terpinene (gamma terpinene; 4-Methyl-1-(1-methylethyl)-1,4-cyclohexadiene) and δ-terpinene (delta terpinene; also known as terpinolene, 1-Methyl-4-(propan-2-ylidene)cyclohex-1-ene) are, like α-terpinene, found in nature and have been isolated from a variety of plant sources.
Gamma terpinene has been found in concentrations of 17% of the oil extracted from dried rosemary. It has a molecular weight of 136.23404 g/mol, and a molecular formula of C10H16.
More to come…
Taxonomy:
- Domain: Eukaryota (yew-carr-ee-OH-tah) — from the Greek prefix ευ (yew) = good, well, pleasing + καρυον (khar-yone) = a nut/nucleus, thus organisms whose cells contain a nucleus and other organelles within membranes.
- (unranked): Bikonta Cavalier-Smith, 1993 (bye-KOHN-tuh) — from the Latin bis = twice/double + the Greek κοντος = a punting pole; those eukaryotic organisms within the subgroups Apusozoa, Rhizaria, Excavata, Archaeplastida, or Chromalveolata.
- (unranked): Archaeplastida Adl et al., 2005 (ahr-kee-PLASS-tih-duh) — from the Greek αρχαιος (AHR-kee-ose) = ancient/antiquated + πλασις (PLAS-iss) = a moulding + Anglo Saxon tid = time; a major group of eukaryotes, comprised of the red algae (Rhodophyta), the green algae, and the land plants along with the freshwater unicellular algae known as glaucophytes.
- Kingdom/Regnum: Plantae Copeland, 1956 (PLAN-tee) or Viridiplantae Cavalier-Smith, 1881 (veer-id-eye-PLAN-tee) — from the Latin planta = a green twig; the plant kingdom, consisting of multi-cellular green plants, i.e., whose cells have cellulose within their cell walls and have primary chloroplasts derived from endosymbiosis with cyanobacteria containing chlorophylls a and b and lack phycobilins..
- (unranked): Streptophyta Jeffrey 1967 (strepp-toh-PHY-tuh) — from στρεπτος (STREP-tose) = (easily) twisted, pliant + φυτον (PHU-tawn) = a plant/tree; the land plants and the green algal group Charophyta.
- Subkingdom: Embryophyta Engler, 1892 (imm-bree-oh-FYE-tuh) — from the Greek εμβρυον (EMM-bree-yon) + φυτον (PHU-tawn) = a plant/tree; green plants, informally known as land plants because most are terrestrial rather than aquatic, while the related green algae are primarily aquatic;
- (unranked): Angiosperms (AN-gee-oh-spurms)/Magnoliophyta Cronquist (mag-NOH-lee-oh-fye-tuh) — from the Greek αγγειον (AUGG-ee-awn) = a vessel/pail/reservoir + σπερμα (SPUR-mah) = a seed; the flowering plants, distinguished from the gymnosperms by having flowers, endosperm within the seeds, and the production of fruits that contain the seeds;
- (unranked) Eudicots (YEW-dee-kotts) — from the Greek prefix ευ (yew) = good, well, pleasing + δι (die/dee) = two/double + κοτυληδων (cott-ee-LEE-dun) = a cup-shaped hollow; a monophyletic clade of flowering plants previously known as tricolpates or non-magnoliid dicots, to emphasize the evolutionary divergence of tricolpat dicots from earlier, less specialized dicots; close relationships are presumed among flowering plants with tricolpate pollen grains (the grains have three colpi, or elongated apertures or furrows in the pollen grain paralleling the polar axis);
- (unranked) Asterids (ASS-tur-iddz) — from the Greek αστηρ (ASS-turr) = a star/meteor + the Latin suffix -idus (EE-duss) = indicative of having the nature of; one of the two most species group of eudicots (which have inflorescences having the appearance of a meteor or shooting star), the other being the rosids;
- Order: Lamiales (lam-ee-AWL-ees) — the etymology of this designation is obscure; comprised of asterids generally having a superior ovary composed of two fused carpels, inflorescences with four petals fused into a tube, bilaterally symmetrical, often bilabial corollas, and four or fewer fertile stamens;
- Family: Lamiaceae (lam-ee-ACE-uh-ee) — the etymology of this designation is obscure; comprised of the mint or deadnettle family of flowering plants containing about 236 genera and some 6,900 to 7,534 species, many aromatic in all parts, others being shrubs, trees (incl. teak), and some vines;
- Subfamily: Nepetoideae (nepp-uh-TOY-duh-ee) — from the Latin noun nepeta = catnip, a kind of mint; a subfamily of the mint family divided into four tribes: 1. Tribe Elsholtzieae, 2. Tribe Lavanduleae (includes lavender), 3. Tribe Mentheae (the largest tribe, containing the herbs sage, thyme, and mint), and 4. Tribe Ocimeae (includes sweet basil);
- Tribe: Mentheae (MEN-thay-ee) — from the Latin noun menthe = mint; comprised of one third of the species of the mint family, Lamiaceae; common names of plants within this tribe are apple-mint, corn-mint, ginger mint, horsemint, mint, orange-mint, pennyroyal, peppermint, spearmint, and watermint;
- Genus: Rosmarinus (roze-MARR-enn-uss) — from the Latin noun ros = dew (also, L. rosa = a rose) + the Latin prep. marinus = of the sea, thus “dew of the sea”; a genus of two to four generally recognized species of perennial herbs with pungent, evergreen, needle-shaped leaves; the two undisputed species, R. officinalis (common in the Mediterranean) and R. eriocalyx (native to northwest Africa and southern Spain) are the best known; R. tomentosus was first recognized in 1941 but is not accepted by some authorities; R. palaui, while first described as a separate species in 2002, is in dispute.
- Species: R. officinalis (oh-feh-kin-ALL-iss) — a fragrant evergreen shrub with needle-shaped leaves
References: In process… we do not post references that are not in our at-hand library and that we have not thoroughly examined. The vetting process takes time. A list of appropriate references relevant to the matter presented in this article is posted below:
- Ozcan, M. M., & Chalchat, J. C. 2008. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr. Vol. 59(7-8):691-8
- Shekarchi, M., et al. 2012. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacognosy. Vol. 8(29): 37–41.
Feel free to e-mail jerry.cates@entomobiotics.com regarding your comments on this article. You may also register, log in, and leave a detailed comment in the space provided below.
